APELOA | Apeloa signs MPP license to make generic Pfizer Covid drug
Apeloa Pharmaceutical has signed a licensing agreement allowing it to produce the full generic version of Pfizer’s oral Covid-19 drug Paxlovid to supply to people in lower- and middle-income countries.
The deal covers 95 designated markets comprising approximately 53% of the world’s population, including various African, South American, South and Southeast Asian countries – but not China.
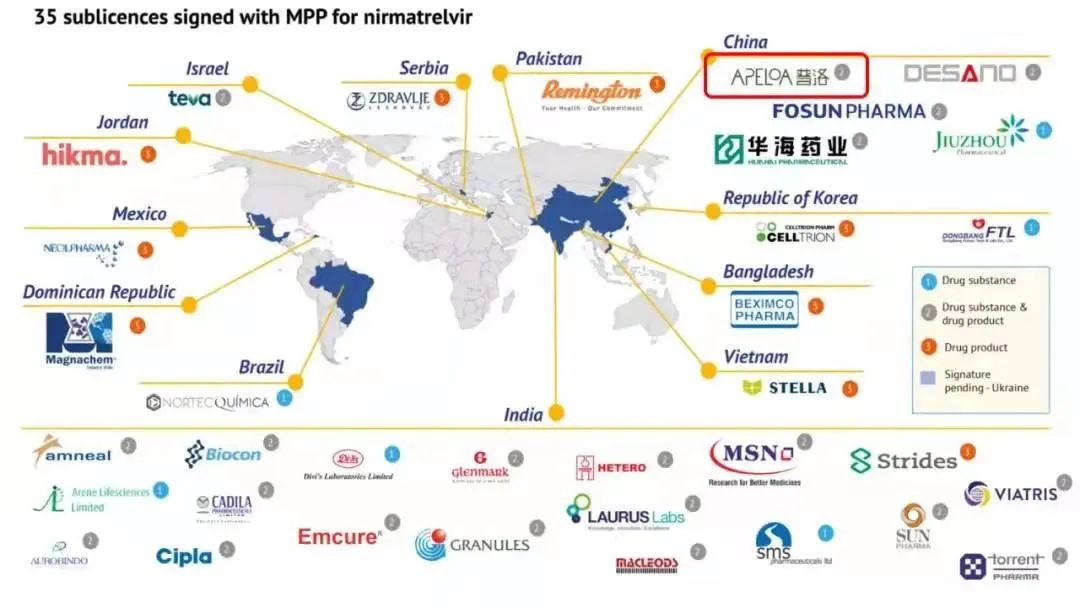
This makes Apeloa one of three Chinese companies that signed with the MPP (Medicines Patent Pool) to make Pfizer’s antiviral treatment nirmatrelvir in combination with low dose ritonavir.
The MPP is a United Nations-backed public health organization that works to access to and facilitate the development of life-saving medicines for lower- and middle-income countries. In November 2021, Pfizer and the MPP had signed a license agreement for Pfizer’s patented Covid-19 treatment.
Pfizer has shown that Paxlovid reduce the risk of death and hospitalization by 89% in high-risk patients when used soon after the onset of symptoms, according to an interim analysis it published in 2021.
Paxlovid has been granted emergency use authorization or temporary authorization in several countries and regions around the world, including approval for condition marketing by the National Medical Products Administration (NMPA) of China in February 2022.
The treatment has also been strongly recommended by the World Health Organization for mild and moderate Covid-19 patients at highest risk of hospital admission. These include unvaccinated, older, or immunosuppressed patients.

According to the agreement between Apeloa and the MPP, Apeloa will manufacture the Covid drug at production facilities approved by the SRA (Stringent Regulatory Authority) or prequalified by the WHO (World Health Organization).
Press Inquiries
E-mail: press@hengdian.com

